Author: Traci Patterson – Founder and Director, Advanced Pathways
There is a big question as to whether a Spinal Cord Stimulator (SCS) is truly the great answer to Complex Regional Pain Syndrome (CRPS) pain that a lot of pain management doctors state that it is.
There is literature and feedback on both sides of this debate. Below is information on what a Spinal Cord Stimulator is supposed to do to help alleviate or decrease the pain for those that have CRPS.
Unfortunately, what they don’t tell you is that there are also articles out there from Neurological Publications that the Neurosurgeons utilize that have shown studies where in some cases SCS have been known to cause the body to build up a layer of scar tissue around the leads or paddle or leads of the SCS. This same scar tissue then continues to grow around the Dura that protects the spinal cord, and if it is not caught it has been known to cause paralysis by putting pressure on the spinal cord. Yes, you read that correctly!
Unfortunately, I have never heard of any doctors or representatives from the SCS companies ever mentioning this to their patients prior to having a Spinal Cord Stimulator implanted. It is never mentioned with the possible risks.
What about the risk of CRPS moving into the back due to the surgery and placement of a SCS? Patients are not told that this can happen and has happened.
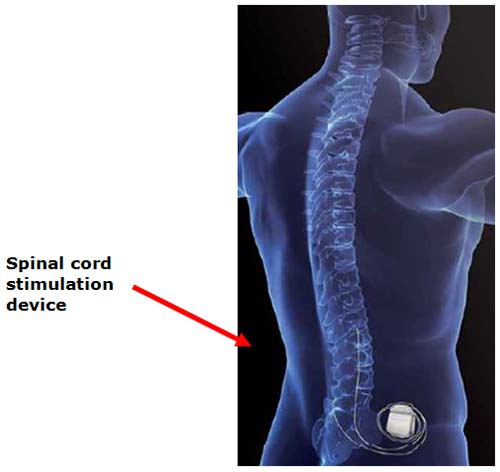
What is a Spinal Cord Stimulator?
When more conservative therapies have failed, the cost and risks of managing pain rise. Increasingly, many healthcare providers are employing a multi-modal approach to pain that includes spinal cord stimulation.
An implantable system delivers electrical pulses via a lead to nerves in the dorsal aspect of the spinal cord. Pain signals are inhibited before they reach the brain and replaced with a tingling sensation (paresthesia) that covers the specific areas where the pain was felt.
A spinal cord stimulation system consists of 2 implanted components:
· Neurostimulator – Rechargeable or non-rechargeable implanted power source that generates electrical pulses according to programmable neurostimulation parameters and features
· Lead – A set of thin wires with a protective coating and electrodes near the tip (percutaneous lead) or on a paddle (surgical lead). The electrodes transmit the electrical pulses to the stimulation site
Two external components to a spinal cord stimulation system allow the therapy to be customized for each patient:
· Clinician Programmer – Used to program the implanted neurostimulator
· Patient Programmer – Empowers patients by giving them a way to manage their pain relief – within preset physician parameters – to optimize outcomes
Other Information:
Prior to having the actual Spinal Cord Stimulator (SCS) implanted a patient will go through what they call a trial. This entails having an external SCS set up with normally 2 leads that are surgically implanted at the correct level on the spine / vertebrae to reduce your pain. The actual SCS itself is outside of your body and kept in a fanny pack that you have to wear, but it gives you an idea if this will work for you.
There have been some patients that have had a very successful trial where the SCS trial decreased their pain levels. Only to have the real SCS that was implanted fail.
There are several manufacturers with this device, most of the time the patient does not have a say in what manufacture is going to be utilized. The Pain Management doctor typically has their preference as to a specific vendor. I highly suggest that anyone that is to the point of considering a Spinal Cord Stimulator to please do your homework. Don’t feel like you have to move forward with this just because your doctor has told your to or has suggested it. There are other options out there to help decrease your pain, and once this has been done it is very difficult to un-do!
Articles and Published Information:
In April 2014, the Wall Street Journal published an analysis of adverse events associated with spinal cord stimulators: “When Spine Implants Cause Paralysis, Who Is to Blame?”
These events were submitted to the FDA or were obtained from medical malpractice law suits. “In many cases, the injuries occurred after patients’ spinal cords were punctured or compressed by the stimulator electrodes….The FDA’s database contains 58 unique reports of paralysis with report or event dates from 2013, compared with 48 in the prior year.” The spinal cord stimulators were made by various companies.
“Researchers at Duke University medical center recently found that nearly one in every 100 spinal stimulator patients experienced some degree of spinal cord or spinal nerve root damage, said Shivanand P. Lad, a Duke Neurosurgeon and the study’s lead researcher. The study, based on insurance claim records of 12,300 stimulator patients has been submitted for presentation at an upcoming medical meeting.”
“A 2011 study based on adverse event reports submitted by device makers found the rate of paralysis or motor weakness in patients implanted with a commonly used type of stimulator was considerably lower, at around 3.8 per 1000, with about 60% of patients eventually experiencing complete or partial recovery.”
Medtronic updated its product label in February to note “that scar tissue can form around device electrodes and cause nerve damage, including progressive quadriparesis, or gradual weakening of all four limbs.”
“Medtronic estimates that as many as 50,000 people in the U.S. are implanted with spinal stimulators each year from all device makers.”
“Stimulators cost between $20,000 and $60,000 each and have estimated global sales of $1.5 billion annually….”
The FDA “cautions that the agency’s database cannot be used to ascertain comprehensive rates of adverse events because the events are under reported [my emphasis] and often contain incomplete or incorrect information.”
The article describes a man with 40 years of back pain who had a spinal cord stimulator implanted at the University of Texas Southwestern Hospital, Dallas. He complained of numbness in his legs. A blood clot was removed on an urgent basis, but damage was irreversible. He was paralyzed from the waist down and left in a wheelchair.
SCS, can cause many more problems that paralysis. They can cause pain, tethering of the cord, scarring of the battery pack that can slide across the back, infection that may cause death, and many other complications. Electrodes may not always be able to be removed and remain permanently scarred into the cord. Deeply troubling is that an MRI can never be done again even if the patient has cancer or stroke —patients either aren’t being told about this or don’t remember being told about this. Where are the five year studies that show benefit? Even with no complications, how long do they continue to relieve pain? Electrodes move and/or they malfunction. There is little to no federal investment in medications that relieve pain, but these devices are garnering sales of $1.5 billion annually without showing lasting benefit. This is a very big source or income for pain specialists, but what is the gain for patients? How can we weight the pros and cons of this money generating device?
So many of the CRPS patients that I have seen failed the small handful of medications now available for chronic pain and were given only one choice by every major pain center: spinal cord stimulator. One choice. This is a very big business but where is the data to support the SCS for CRPS? What about all the negative outcomes, the patients that have their CRPS move into the surgical areas following the implant of a SCS or worse?
It is important for all CRPS and chronic pain patients to know their options and to be informed about risks vs rewards of all treatments. There are other options available beyond a SCS. These are non-invasive and drug-free options that are providing long-term relief for CRPS patients. Know your options.
For additional information or if you would like to speak with me please contact me at: Info@AdvancedPathways.com